
Experiments
This is an overview of the experiments.
Details of principles and procedures are explained in the textbook.
Laboratory 1
Reactions of Inorganic Metal Cations
The aim of this experiment is to understand the equilibrium of the inorganic reactions such as precipitation of metal salts and formation of metal complexes, and to learn the systematic method to separate and to confirm metal ions in a solution. In this experiment, you will isolate the specific metal ions as precipitates by adding appropriate reagent. Isolated metal ion is dissolved into solution and qualitatively analyze the existence of the ions by characteristic coloration reactions.
→Textbook: Section 2.1, 2.2
Movies: Procedures
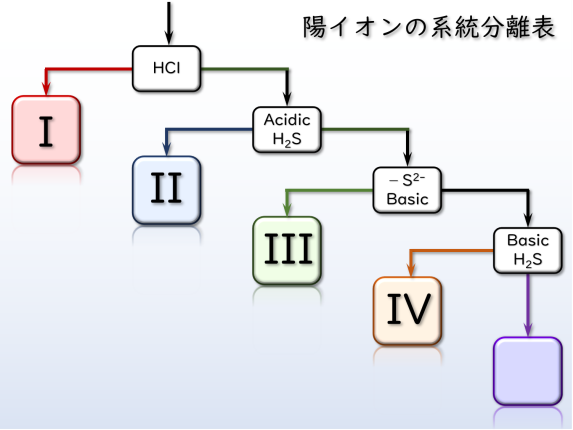
Qualititative Analysis of Metal Cations
Based on the knowledge of the “Reactions of Inorganic Metal Cations”, you will isolate metal ions from the solution includes different kinds of metal ions and confirm them by characteristic reactions. In the experiment, each student will be provided with a sample solution containing various combinations of metal ions. Enjoy!
→Textbook: Section 2.3
Laboratory 2
Synthesis of Acetylsalicylic Acid
In this experiment, you will synthesize acetylsalicylic acid. Acetylsalicylic acid is well known as aspirin which is a medication used to reduce pain, fever or inflammation. You will purify acetylsalicylic acid by recrystallization and will learn how to measure its melting point.
→Textbook: Section 3.1
Movies: Procedures, Crystallization
Synthesis of Iron Complex
In this experiment, you will synthesize potassium iron(III)oxalate and purify it by recrystallization. Iron(III)oxalate complex is known to decompose and produce iron(II) ion under visible light irradiation. Yow will perform a photochemical reaction and learn how to detect produced iron(II) ion in the solution.
→Textbook: Section 3.2
Movie: Procedure
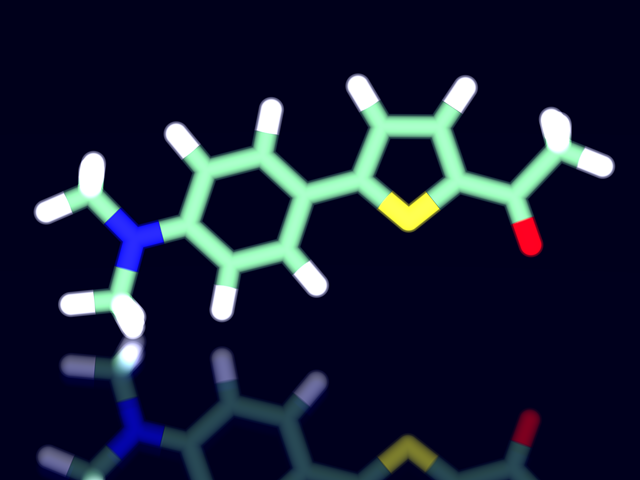
Synthesis of Fluorophore
In this experiment, you will synthesize a phenylthiophene derivative by Suzuki-Miyaura coupling reaction. During the reaction, you can check the progress of the reaction by thin layer chromatography (TLC). Synthesized phenylthiophene derivatives show fluorescence when UV light is irradiated. The color of fluorescence strongly depends on the polarity of the solvent. Various fluorescent color under UV irradiation is impressive.
→NOT included in the course nor the textbook.
Movies: Procedure, Solvatochromism
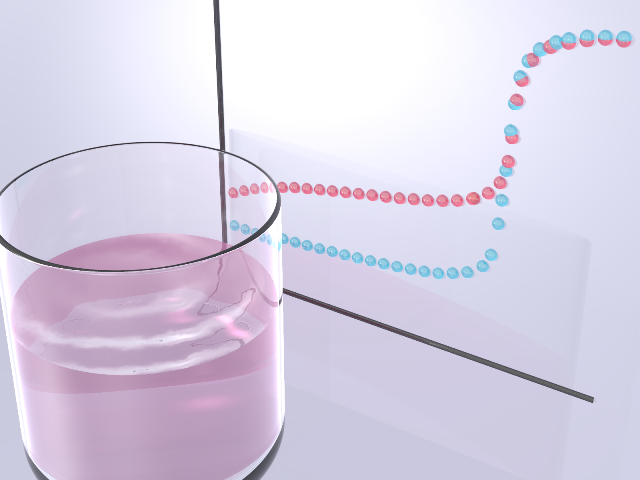
Titration
The aim of this experiment is to learn quantitative analytic method of acid-base reaction. You will titrate two acid solutions hydrochloric acid and acetic acid with sodium hydroxide solution under measuring pH of the solutions. From the data, concentration of the acid, acid dissociation constant can be analyzed.
→Textbook: Section 4.1
Movie: Procedure
Laboratory 3
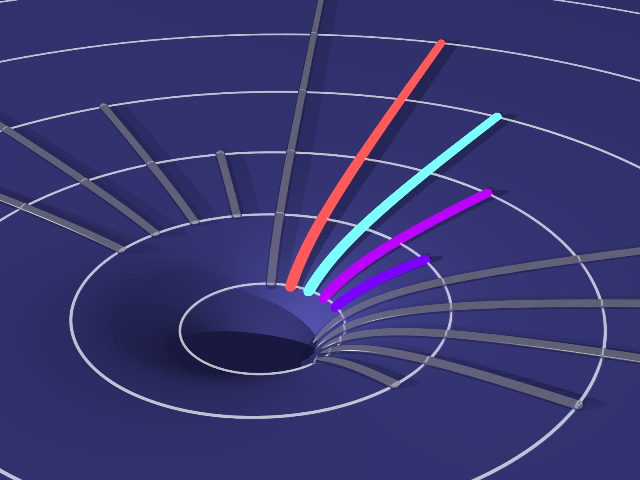
Spactra of Various Light Sources
In this experiment, you will observe the spectra of various light sources such as hydrogen discharge tube, sodium lamp, incandescent lamp, fluorescent lamp and LED. You will see different feature of spectra due to different mechanisms of emission. You will be able feel a hint of quantum mechanics.
→Textbook: Section 5.1
Movie: Procedure
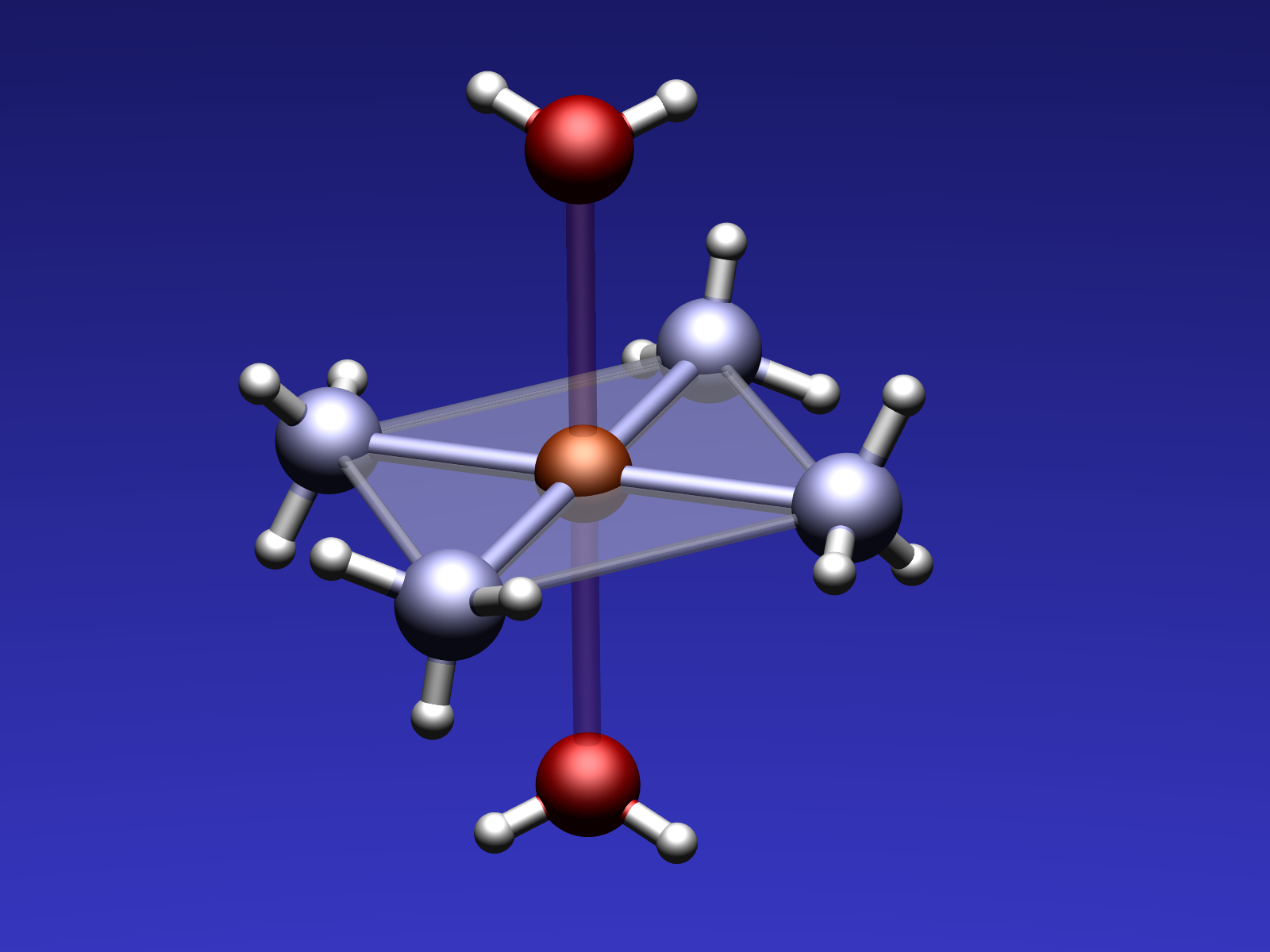
Absorption Photometry
In this experiment, you will study the relationship between the absorption and the concentration of a colored solution which is stated by Based on the Beer-Lambert law. You will learn how to make a calibration curve from a set of absorption measurements on tetraamine copper complex standard solutions in various different concentrations. With the calibration curve, you will analyze a concentration of an unknown sample.
→Textbook: Section 5.2.2
Movie: Procedure
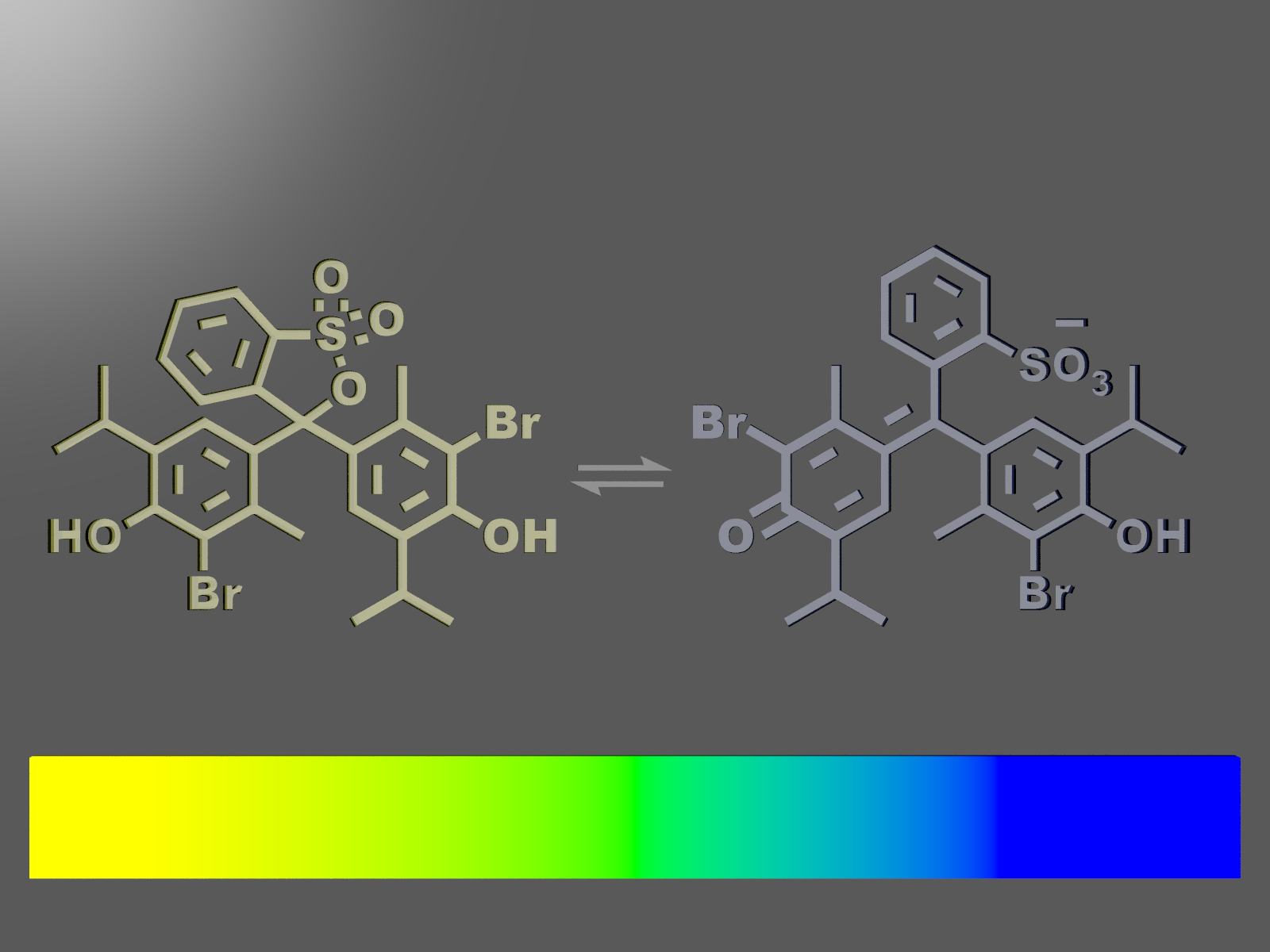
Absorption Spectra of Molecules
In this experiment, you will observe the colors and spectra of phenolphthalein solution in acidic and basic conditions. (In this year, the following experiment on BTB molecule will be demonstrated instead.)
→Textbook Section 5.2.1
In this experiment, you will measure absorption spectra of bromothymol blue (BTB) solutions in various pH. Through the analysis of spectra, you will learn the basic idea of equilibrium of acid dissociation reaction in a solution.
→Additional document
Movie: Procedure
Chemical Oscillation Reaction
In this experiment, you will observe Belousov-Zhabotinsky (BZ) reaction. The color of the reaction solution changes periodically. You will analyze the oscillation intervals measured at various temperatures and estimate the activation energy of the reaction.
→Textbook: Section 6.1
Movie: Procedure